Dilated Cardiomyopathy Mimicking Arrhythmogenic Right Ventricular Cardiomyopathy in a 35-Year-Old Woman
Article information
Abstract
While dilated cardiomyopathy (DCMP) typically involves the left ventricle (LV), it can also impact the right ventricle (RV). Arrhythmogenic right ventricular cardiomyopathy is a heritable myocardial disorder that mainly affects the RV. A 35-year-old woman presented to the emergency department complaining of chest discomfort and palpitations. The patient had ingested 100 g of alcohol daily for more than 5 years. Transthoracic echocardiography showed dilation of the four cardiac chambers with global systolic dysfunction of the prominent myocardial recesses of both ventricles. Non-sustained ventricular tachycardia was observed on 24-hour Holter monitoring. On magnetic resonance imaging (MRI), the ratio between noncompacted and compacted layers did not meet the criteria for non-compaction, and delayed gadolinium enhancement was only observed in the inferior LV myocardium. Endomyocardial biopsy revealed no fibrosis or fatty changes. Consequently, a diagnosis of alcohol-induced DCMP was reached. Various diagnostic modalities, including MRI and biopsy, can contribute to accurate diagnoses.
INTRODUCTION
Right ventricle (RV) dysfunction can be observed in various cardiomyopathies including dilated cardiomyopathy (DCMP) or arrhythmogenic right ventricular cardiomyopathy (ARVC). ARVC is one of the leading causes of arrhythmic cardiac arrest in young people and athletes [1]. DCMP mainly causes left ventricular (LV) dilatation and contractile dysfunction but may also involve the RV with a worse prognosis [2]. Secondary trabeculation due to LV remodeling in DCMP is morphologically similar to LV non-compaction, making it difficult to differentiate [3,4]. We present the case of a woman with right ventricular dysfunction with arrhythmic events and ventricular dysfunction with a prominent recess.
CASE
A 35-year-old woman presented to the emergency department. She complained of recurrent episodes of palpitation beginning 1 week prior. She had marked limitations in physical activity (functional classification III of the New York Heart Association [NYHA]). The patient had an unremarkable medical history. She had consumed more than 100 g of alcohol (two bottles of Soju) per day for more than 5 years and was a current smoker with a 15-pack-year history. She denied a family history of premature sudden death or cardiomyopathy.
On physical examination, initial blood pressure was 130/97 mmHg, heart rate was 187 beats per minute, body temperature was 36.7℃, and respiration rate was 25 breaths per minute. In room air, the oxygen saturation was 98%. The lung sound was coarse with bilateral crackling. Heart sounds were regular with an insignificant murmur. Grade 2 peripheral pitting edema was observed in both legs.
Initial electrocardiography revealed supraventricular tachycardia (Fig. 1A). Six and 12 mg of intravenous adenosine at 7-minute intervals were administered followed by a saline flush. Sinus conversion was achieved after injection. No bundle branch blocks and ventricular premature complexes were observed. No epsilon wave was seen in lead V1 and V2. T wave was not significantly inverted in the limb or precordial leads (Fig. 1B).

Electrocardiogram on initial presentation. (A) Electrocardiogram taken during palpitation shows narrow QRS tachycardia with a regular heart rhythm, presenting paroxysmal supraventricular tachycardia. (B) After intravenous adenosine, normal sinus rhythm is noted.
On laboratory examination, blood cell count analysis revealed mildly increased white blood cells (11,200/mm3, 78% segmented neutrophils) with normal hematocrit and platelet counts. Prothrombin and partial thromboplastin times were normal. D-dimer was slightly increased by 0.66 mg/L, while the natriuretic pro-brain natriuretic peptide value was 10,069 pg/mL and lactic acid was 3.0 mmol/L. Creatine kinase with MB isoenzyme and troponin-T were within the normal range. Liver and kidney function tests revealed no abnormalities, and urine analysis was insignificant. On arterial blood gas analysis, no acidosis or hypoxia was observed. Plain chest radiography revealed cardiomegaly and bilateral pulmonary congestion.
On transthoracic echocardiography, LV was markedly dilated (LV end-diastolic dimension was 62 mm and indexed dimension was 39 mm/m2). The LV wall was diffusely thickened by 13 mm. Global LV hypokinesis with severe systolic dysfunction (ejection fraction [EF] by modified Simpson’s method was 14%) was noted. Significant trabeculation with a deep recess was noted throughout the LV myocardium (Fig. 2). The maximum ratio of noncompacted to compacted myocardium was 2.1. Peak E and A velocities of mitral inflow were 88 and 41 cm/sec, respectively (E/A ratio was 2.14). The peak velocity of the septal mitral annulus (E’) was 6.8 cm/s and E/E’ ratio was 13. The RV was dilated (basal and mid RV dimensions at end-diastole was 43 and 31 mm, respectively) with normal wall thickness. The systolic excursion velocity of the RV free wall (S’) was 10.8 cm/sec with a fractional area change of 12.1%, indicating RV systolic dysfunction. RV wall motion was diffusely impaired, satisfying the major imaging criteria of the international task force criteria of ARVC [5,6]. Mild functional mitral and tricuspid regurgitation were noted. Systolic pulmonary arterial pressure was 59 mmHg. The diameter of the inferior vena cava was 23.2 mm and plethoric.

Transthoracic echocardiography. In an apical four-chamber view (A) and a parasternal short-axis view (B) at end-diastole, prominent trabeculations and deep recesses are noted at the left ventricle (arrows). The maximum ratio of non-compacted to compacted myocardium is measured at 2.1.
To rule out secondary causes of DCMP, various additional tests were performed. Thyroid function, autoimmune markers, human immunodeficiency virus antigen & antibody, and complements levels were within normal limits. On 24-hour Holter monitoring, while basic sinus rhythms were noted, burdens of ventricular premature complexes (12%) and nonsustained ventricular tachycardia originating from the LV were noted, which satisfied the minor criteria of the international task force criteria of ARVC.
Cardiac magnetic resonance imaging (MRI) with gadolinium enhancement was completed. There was dyssynchronous movement of LV and dyskinetic RV on the cine images. Global akinesia of the LV and RV walls was also noted. The LV was dilated (62 mm at end-diastole) and the wall was diffusely thinned by approximately 5.6 mm. LV EF and RV fractional area changes were 19% and 20%, respectively. Myocardial perfusion was preserved on perfusion imaging. Regional, transmural, and patchy delayed gadolinium enhancement was detected in the junctional area of the inferior LV wall. The end-diastolic ratio between the non-compacted (red line) and compacted (green line) layers was less than 2.3. Therefore, we could exclude isolated LV non-compaction since a maximum end-diastolic myocardial thickness ratio was less than 2.3 on the MRI image (Fig. 3) [7].
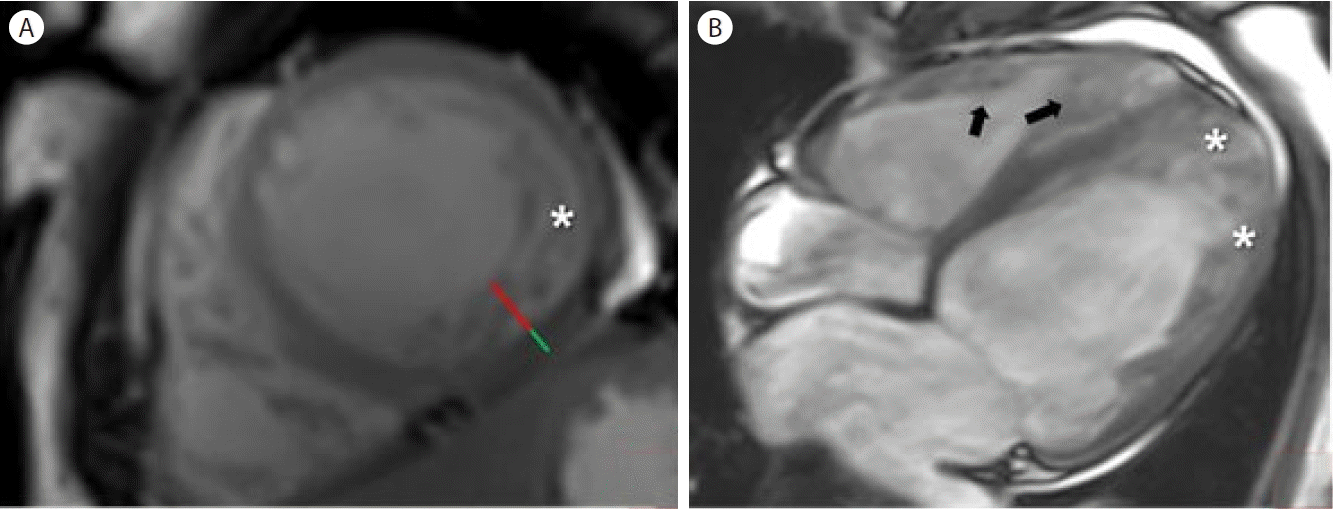
Cardiac magnetic resonance imaging (MRI). (A) Short-axis cine steady-state free precession (SSFP) cardiac MRI at end-diastole shows prominent trabeculations (asterisk). The end-diastolic ratio between the non-compacted (red line) and compacted (green line) layers is measured at less than 2.3. (B) End-diastolic long-axis cine SSFP cardiac MRI reveals prominent trabeculations (asterisks) in the left ventricular lateral wall and their extension to the apex. Prominent trabeculations of the right ventricle (arrows) are also appreciated.
To distinguish between ARVC and DCMP, we decided to perform an endomyocardial biopsy for endomyocardial tissue characterization. The catheter was inserted into the RV through the right internal jugular vein. Myocardial tissue was obtained from the apical interventricular septum using biopsy forceps. To obtain optimal tissue, transthoracic echocardiography was used as a guide for the myocardium with deep recess. On Hematoxylin and Eosin staining, the concentration of myocardium was within normal range and there was no fatty deposition or interstitial fibrosis, which did not meet the feature of ARVC and non-compaction. Accordingly, the final diagnosis of the presented case was DCMP after the exclusion diagnosis of LV non-compaction and ARVC. The etiology of cardiomyopathy was presumed to be alcohol.
The patient was treated for the management of heart failure with reduced EF. After her symptoms such as orthopnea and pretibial pitting edema improved by intravenous loop diuretics, a low-dose angiotensin receptor blocker was added. After pulmonary congestion had been resolved, beta-1 selective blockers were added, and it was determined that the patient should be discharged and switched to outpatient treatment. After one month, the patient visited the hospital. Although she complained of shortness of breath on exertion, NYHA functional class was improved by one. We maintained the treatment and prepared a future plan to insert an implantable cardioverter defibrillator. However, the patient has yet to revisit our hospital since.
DISCUSSION
DCMP is a type of myocardial disorder that typically involves the LV. Although half of DCMPs are idiopathic, excessive alcohol consumption is also known to cause DCMP [8]. The pathogenesis of alcoholic cardiomyopathy is not well understood. Ethanol and acetaldehyde metabolites are known to induce mitochondrial dysfunction, oxidative damage, and impaired calcium ion hemostasis, damaging the myocardium [9]. The most important things when suspecting alcoholic cardiomyopathy are a long history of heavy alcohol intake, evidence of LV dilation, and symptoms of heart failure. The diagnosis of alcoholic cardiomyopathy can be made if other causes are excluded. Most studies have shown that the amount of alcohol required to establish alcoholic DCMP is at least 80 g consumed daily for at least 5 years [10,11].
Ischemic heart disease is the most common cause of heart failure [12]. Although diffuse hypokinesis and systolic dysfunction were present on initial echocardiography, the likelihood of coronary disease was considered to be low because the patient was too young and had no underlying disease, and cardiac enzyme levels were within the normal range. In addition, there were no findings suggestive of ischemic heart disease on cardiac MRI. Therefore, coronary evaluation was not performed.
ARVC exhibits an autosomal recessive genetic disorder in about half of cases and is known as a disease in which RV origin ventricular tachycardia occurs due to fatty deposition in the RV [13,14]. Global or regional dysfunction and structural alternations including regional RV akinesia, dyskinesia, or aneurysm could be determined by echocardiography, MRI, or RV angiography [1,6]. ARVC is a disease that progresses over time, and it is known that it is not limited to RV involvement, but also LV [15]. In addition, LV lesions are associated with clinically significant arrhythmias and more severe heart failure with higher mortality [14,16]. In this case, regional RV akinesia with RV systolic dysfunction (fractional area change ≤ 33%) on MRI as major criteria and arrhythmias with numerous ventricular extrasystoles on Holter as minor criteria satisfied the borderline criteria of ARVC.
LV non-compaction can be diagnosed when the ratio of a thin compacted myocardium and thick trabeculated myocardium is greater than 2.1 at end-systole in the parasternal short-axis view, or when the maximum end-diastolic noncompacted to compacted myocardial thickness ratio is greater than 2.3 at end-diastole [17]. However, secondary trabeculation due to LV remodeling in DCMP may also have non-compaction phenotypes, making it difficult to distinguish between existing criteria [3,4].
The long period of alcohol intake, symptoms of heart failure, and echocardiographic findings suggested the possibility of alcoholic DCMP for this patient [18]. Since non-sustained ventricular tachycardia was noted on Holter monitoring and bilateral diffuse akinesia was observed at the RV on echocardiography, it was necessary to exclude ARVC. In addition, initial echocardiographic findings of multiple trabeculations and deep recesses suggested the possibility of LV non-compaction. Therefore, a cardiac MRI was performed for differential diagnosis. A non-compaction to compaction ratio less than 2.3 on MRI could exclude the diagnosis of LV non-compaction.
There are a few limitations to our case report. First, for the differential diagnosis of ARVC and DCMP, we obtained a tissue sample from the affected area at the septum rather than the RV free wall since the RV wall was too thin and was at risk of perforation. However, the most informative region for pathology is known to be the antero-apical wall, and the septum or LV has been reported without significant diagnostic value [18]. Second, immunohistochemistry could not be performed because of insufficient myocardial tissue. Third, for further evaluation, we offered the patient a genetic test for ARVC. However, she turned it down because of financial concerns. Fourth, although we planned to perform echocardiography to see the improvement of EF, the patient was lost to follow-up after the second outpatient visit. Therefore, further management including medications for heart failure with reduced EF and implantable cardioverter-defibrillator implantation was not possible.
In conclusion, it could be difficult to diagnose DCMP with right heart involvement from another cardiomyopathy, such as ARVC and LV non-compaction. Various modalities including MRI and biopsy can aid in accurate diagnosis.