INTRODUCTION
Over the past years, speckle tracking-derived global longitudinal strain (GLS) has been validated for the assessment of left ventricular (LV) function [1]. It provides additional and more sensitive information about myocardial performance, particularly in the early or subtle stages of cardiac dysfunction [2]. Studies have also demonstrated that GLS is a strong predictor of adverse events in various spectra of cardiac disease, such as heart failure, ischemic heart disease, arrhythmia, and valvular heart disease [3-8]. The aim of this review is to explore the clinical application of GLS.
THE BASIC CONCEPT OF SPECKLE-TRACKING-DERIVED LEFT VENTRICULAR STRAIN
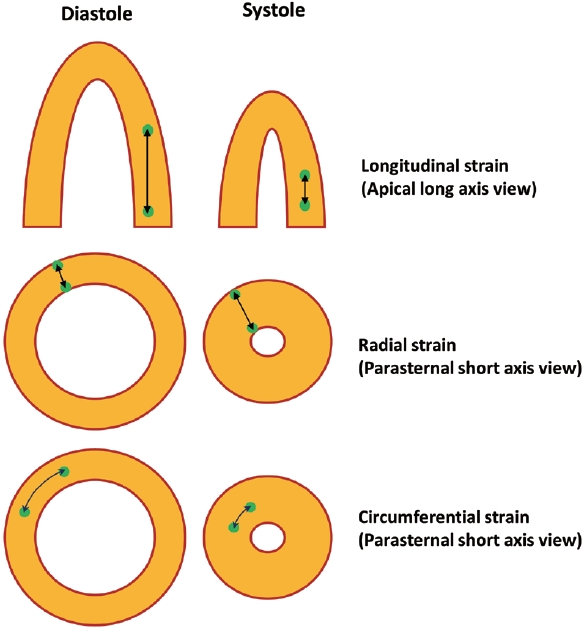
Speckle-tracking derived strain is a technique for the assessing myocardial deformation (changes in shape and size) of the myocardium during the cardiac cycle. Speckles are small, natural acoustic markers within the myocardial tissue appearing as random, grainlike patterns on echocardiographic images. The speckle-tracking software identifies and tracks these speckles frame by frame as the heart beats. The software measures the relative displacement of speckles between frames, enabling the quantification of deformation. This deformation information is expressed as strain, which is a dimensionless unit representing the percentage change in the length of a segment of tissue. Strain can be assessed in various directions, including longitudinal (along the long axis of the heart), circumferential (around the circumference of the heart), and radial (perpendicular to the heart's surface) (Fig. 1). Global strain represents the average strain across the entire myocardium, allowing for an overall assessment of cardiac function. In particular, longitudinal strain has proven to be useful in clinical practice. The reference values for GLS can vary depending on the vendor or manufacturer of the echocardiography equipment and the specific software used. While it may vary depending on the manufacturer, the normal range of GLS is commonly reported to be between -18 and -21 [9].
GLOBAL LONGITUDINAL STRAIN IN THE ASSESSMENT OF LEFT VENTRICULAR SYSTOLIC FUNCTION
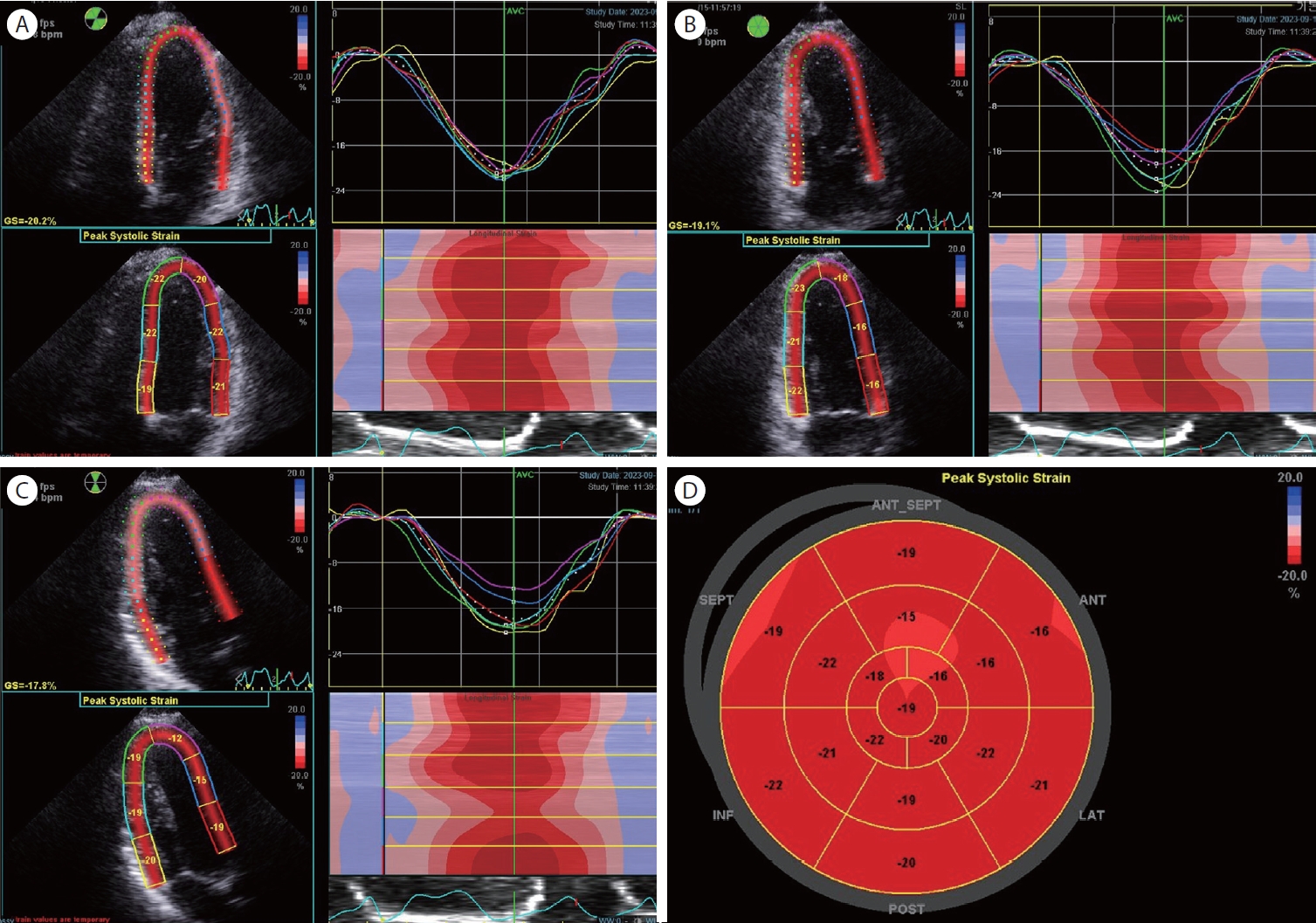
GLS provides a quantitative assessment of global LV systolic function. It measures the percentage change in myocardial length during the cardiac cycle, representing the overall longitudinal shortening or lengthening of the LV. Currently, two-dimensional speckle-tracking echocardiography is widely utilized in clinical settings. Achieving high-quality images is the initial step for precise measurements. GLS assessment of the left ventricle involves capturing apical two, and four chambers view and apical long-axis view (Fig. 2). Achieving an optimal image is the initial step in precise GLS measurement. The operator should manipulate the region of interest (ROI), representing the LV cross-sectional shape, to align with the cross-section of the myocardium throughout the cardiac cycle. The thickness of the ROI can also be manually adjusted to match the thickness of the patient's myocardium. Ensuring the precise identification of two reference points in every region throughout the cardiac cycle is essential for successful tracking and subsequent analysis. These reference points were the end of diastole and systole. Automated detection algorithms are typically helpful, but it is important for the operator to verify that the points are accurate. Specialized software is used for GLS analysis, with each vendor offering their unique strain analysis tools, leading to some vendor-specific variations in GLS reference values.
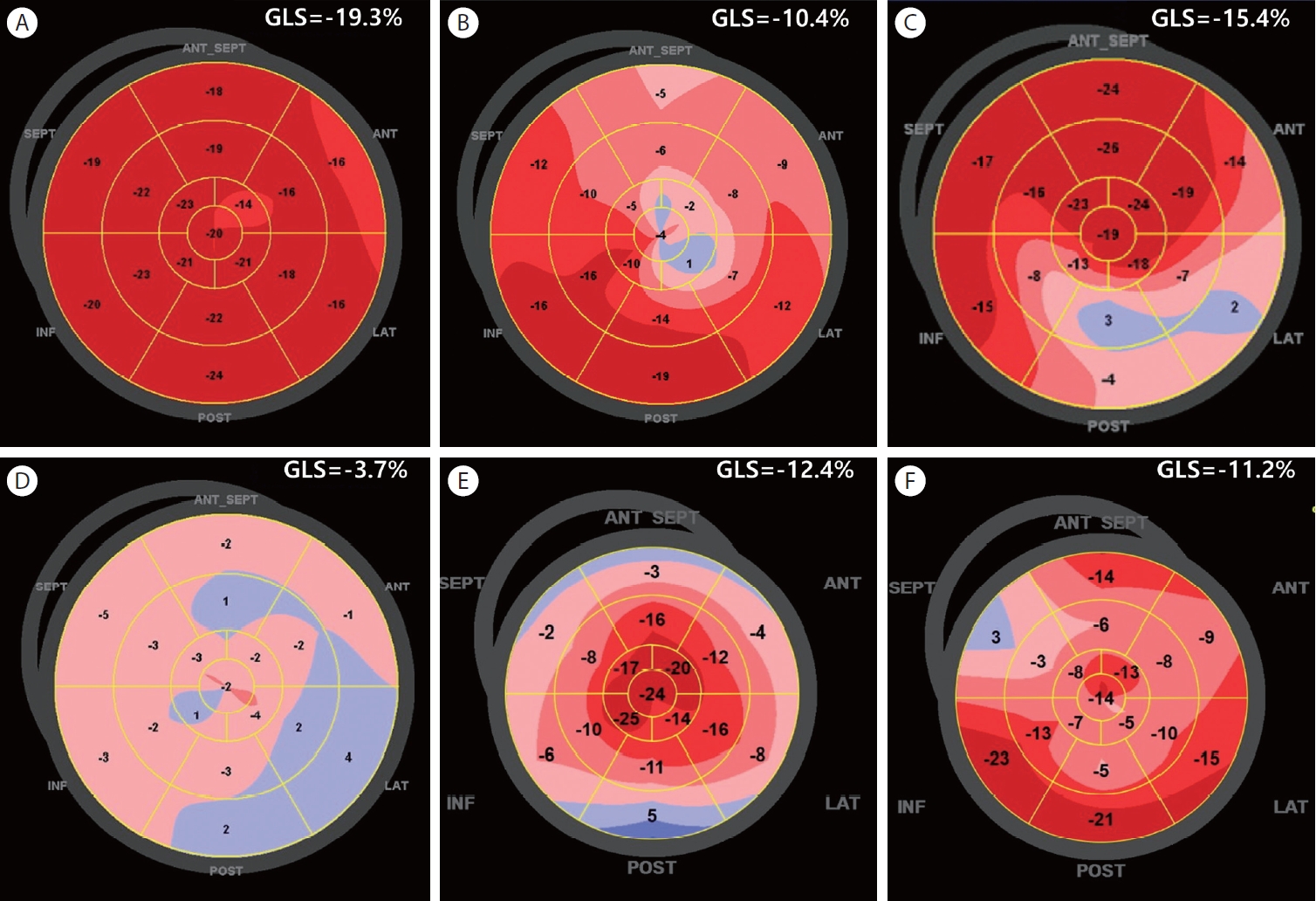
GLS exhibits high sensitivity to subtle changes in myocardial function and can detect abnormalities in LV function at an earlier stage than ejection fraction (EF), making it particularly valuable for identifying early stages of heart disease [10]. Many studies also have shown that abnormal GLS values can provide prognostic information beyond what EF offers in those various spectrums o f h eart d isease [10,11]. R egional s train i s a lso presented through a bull’s eye map (Fig. 3), allowing for a quick visual assessment of the regional distribution at a glance.
ROLE IN HEART FAILURE AND CARDIOMYOPATHY
GLS can detect abnormalities before they become evident through changes in traditional parameters such as EF [12]. This makes it particularly valuable for the early detection of heart failure and monitoring of its progression, which can result in early intervention and management, potentially preventing overt myocardial damage. Recent studies have demonstrated that GLS can sensitively detect early LV dysfunction in patients with chemotherapy-induced cardiomyopathy, which may not be identified by left ventricular ejection fraction (LVEF) alone [13,14]. The prognostic value of GLS has been validated in patients with acute and chronic heart failure [3,15]. Although GLS is a parameter of systolic function, impaired LV GLS is common in patients with heart failure with preserved EF [16], and is also related to clinical outcomes in such patients [17].
The regional strain by bull’s eye map can be useful in various types of cardiomyopathy. LV wall thickness and structural changes are associated with GLS. In patients with hypertension, wall stress by pressure overloading, microvascular dysfunction, myocardial ischemia, and myocardial fibrosis are related to impaired GLS. Poor GLS was associated with adverse outcomes i n t he s e pat ient s [18]. In pat ient s wit h hy p er t roph ic cardiomyopathy, impaired GLS was associated with impaired wall thickness. The geographical distribution of LV hypertrophy aligns well with the regional GLS impairment assessed using the bull's eye map (Fig. 3). GL S also holds prognostic significance across various types of cardiomyopathy, exhibiting higher sensitivity or providing additional value compared with LVEF [10,18,19]. In cardiac amyloidosis, the bull’s eye pattern of GLS can provide a diagnostic clue, which is an apical sparing pattern (Fig. 3).
THE ROLE IN ISCHEMIC HEART DISEASE
GLS is highly sensitive to subtle changes in myocardial function. This is particularly important for the early identification of ischemic damage. This can be applied to the assessment of regional myocardial function. It can specify areas of the myocardium that may be experiencing dysfunction, which helps assess the regional function of LV in ischemic heart disease [20]. Regional wall motion abnormalities detected by using the bull’s eye map of GLS are presented in Figure 3. Figure 3B and 3C demonstrate the detection of the extent and location of myocardial infarction. During stress echocardiography via exercise or pharmacologic modalities, the angina pectoris can also be evaluated by measuring regional strain in addition to visual assessment of regional wall motion abnormalities [21]. Aside from the diagnosis of ischemic heart disease, it can aid in the decisionmaking process for the revascularization procedures. GLS values are also strong predictors of adverse cardiac events [22].
ROLE IN VALVULAR HEART DISEASE
LVEF has a limitation that it does not consider the direction of blood flow, and therefore, it cannot calculate the effects of valvular regurgitation. GLS is less affected by the valvular regurgitation and less dependent on preload and afterload compared with LVEF. As a result, GLS can provide a more accurate assessment of LV contractility than LVEF, which may overstate LV function, particularly under certain valvular conditions such as mitral regurgitation. The sensitivity of GLS in the detection of early myocardial dysfunction also allows for timely intervention before significant deterioration occurs in the myocardium. Major society guidelines highlight the usefulness of GLS in valvular heart diseases because GLS can provide extra insights for patients with aortic regurgitation, aortic stenosis, and mitral regurgitation, offering prognostic information during follow-up and post-surgery, in addition to LVEF [22-25]. In patients with moderate to severe mitral regurgitations undergoing mitral repair, good GLS values have been shown to predict better long-term LV systolic function [26]. LV hypertrophy is common and LVEF can be overestimated in aortic stenosis. GLS has exhibited a close correlation with post-operative prognosis and can be a better prognostic parameter than LVEF [27].
LIMITATIONS OF GLS
One limitation of GLS is its dependency on image quality. Poor image resolution or inadequate acoustic windows can hinder the accurate measurement of myocardial deformation, potentially leading to less reliable results. In addition, GLS may be influenced by technical factors, such as frame rate and the specific software used for analysis, which can introduce variability in measurements. Another limitation is the need for standardized guidelines and reference values, as different vendors and software platforms may use slightly different methodologies for calculating GLS. Furthermore, GLS primarily a ssesses long it ud ina l fu nction a nd may not provide a comprehensive eva lu ation of other a spect s of ca rd iac performance, such as radial or circumferential strain. Clinicians need to be mindful of these limitations when interpreting GLS data in clinical practice.
CONCLUSION
GLS has emerged as an important clinical tool for myocardial function assessment. It measures the deformation of the heart muscle along its longitudinal axis and provides valuable insights into myocardial performance. By analyzing the deformation of the heart muscle fibers, GLS offers a sensitive indicator of cardiac contractility, making it particularly useful in the early detection and monitoring of various cardiac conditions, such as heart failure and cardiomyopathies. In addition, GLS is highly reproducible and less operator-dependent compared to traditional echocardiographic parameters, enhancing its reliability in clinical practice. It enables clinicians to detect subtle changes in cardiac function that may be overlooked in conventional assessments, such as LVEF. Furthermore, GLS has shown promise in predicting adverse cardiovascular events and guiding therapeutic decisions, making it an indispensable tool for optimizing patient care and outcomes in cardiovascular imaging.