Introduction
Successful breast cancer surgery is identified as excision with negative margins. Ultrasound (US) examination is a preferred method in localizing mammographically occult breast lesions. It is necessary to place a guide wire locating the tumor before the operation and assess the surgical specimen with specimen radiography intra-operatively. In that way, it is possible to confirm the excision of the non-palpable lesion and tumor-free margins [1,2]. Specimen radiography is one of the methods that helps in ameliorating re-excision rates [3-8].
US and mammography are the modalities that are used intra-operatively for specimen radiography [8-15]. Although mammography of the specimen is the conventional method informing these aims, handheld US (HHUS) proved to be an alternative, particularly in mammographically occult cases where US is the main detection modality. Lesions that are US detected per se but not visible on mammography are localized by US-guided wire placement. In such lesions, US becomes the essential modality for evaluation of the excised specimen [9-14]. Specimen US is a proven time and cost saving modality which has shown promise in ensuring the histological surgical margins of particularly US only detected lesions [12-15]. In this technical note, we want to share our experience with automated 3D breast US (ABUS) in specimen imaging and its practicability.
Case
A 52-year-old woman presented with 3 solid lesions in a linear trace of the ductal system in the upper outer quadrant (UOQ) of the right breast. The lesions were well circumscribed, hypoechoic, round in shape and measured 6.7, 4.5 and 5.4 cm in diameter respectively. Her two-view mammogram did not show the lesions which were non-palpable in clinical breast examination.
US-guided vacuum aspiration biopsy was performed and intraductal papilloma fragments were identified in the specimen. The lesion diagnosis was papillomatosis, leading to a segmental excision. She underwent US-guided wire localization before the excision and the wire location was confirmed with online US imaging before the operation and the location of the tip of the wire was marked on the skin in order to guide the surgeon.
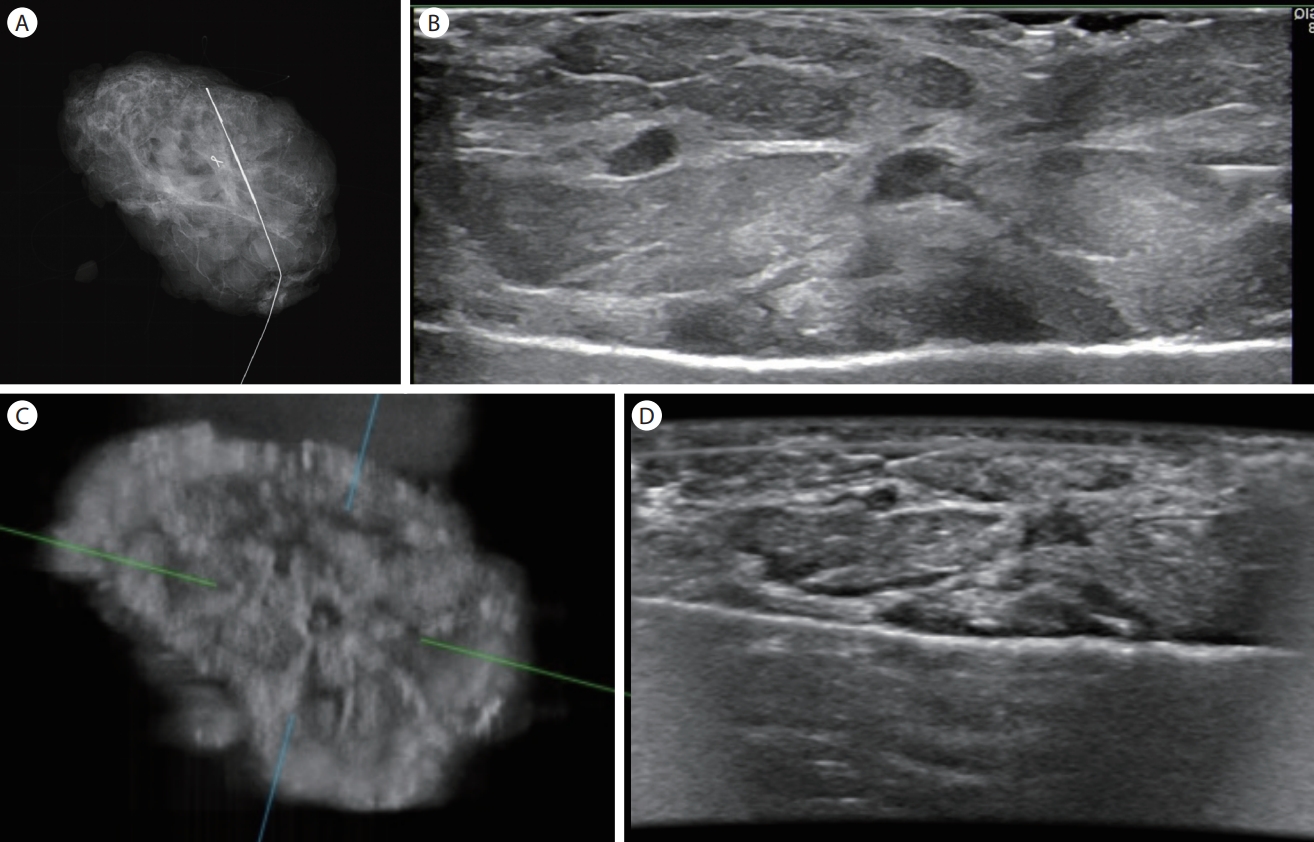
The excised segmental mastectomy specimen was taken in a plastic box to the radiology department. The specimen radiography was taken with mammography (Fig. 1A). Afterwards it was evaluated with HHUS (Fig. 1B) followed by an ABUS examination (Fig. 1C and D).
For ABUS examination, the specimen was placed on a metal tray identically as it was placed on the mammography detector or for HHUS imaging. The specimen was then covered with stretch wrap film. A thin layer of US gel was applied on the stretch wrap film and the mesh membrane covering the head of the ABUS transducer holder was placed over the stretch wrap covered specimen. The scan head assembly holding 15-inch transducer was pressed with the help of the compression assist feature of the device and the first level of three levels of compression was set. The specimen was placed in the center of the scan head assembly and sweeping the area of the transducer and the acquisition of 1-minute scan was initiated. The raw data of the scanning volume was evaluated on a separate workstation. Coronal, transverse, and oblique views of the specimen were generated. These images were compared with the 2 view mammography images and HHUS transverse images which were obtained before the ABUS acquisition. The images of all three modalities were compared by two radiologists. The presence of the lesion within the resected tissue was verified and the relationship of the lesion and the surrounding healthy tissue was evaluated. ABUS showed better delineation of the lesion margins in coronal views (Fig. 1C). Although HHUS had better resolution in transverse images, the transverse ABUS images were comparable (Fig. 1B-D). The histopathologic result was papillomatosis with dysplastic alterations. Surgical margins were clean.
Discussion
Confirmation of the lesion in the excised segment is the main purpose of a non-palpable breast lesion, while the information on the surgical margin proximity being the second one [3-6]. Although mammography of the specimen is the conventional method sustaining these aims, HHUS is proved to be an alternative particularly in mammography occult cases where US is the main modality used for detecting those [9-15]. This is an intra-operative examination that needs to be fast and easy to reach and an expert should be present for the acquisition and evaluation of the real time HHUS. ABUS, on the other hand, can be easily performed by a sonographer and does not need the presence of the radiologist during the examination. The volume data can be evaluated in a separate remote workstation by a radiologist, and the data can be archived that allows the re-evaluation of the study in the need of a comparison with the gross pathology or histopathology examinations. Coronal images are one of the advantages of ABUS in delineating the spiculations and retraction of the lesions. It can be more informative in understanding the relation of the lesion with the surgical margins. Furthermore, delineation of intraductal components can be problematic most of the time due to an absence of contrast between the lesion echo texture and its surrounding healthy tissue. However, coronal images are superior for detection of these intraductal components [16,17].
In conclusion, we believe that ABUS examination of the surgical specimen, particularly in US-only detected lesions, may be as reliable and practical HHUS. ABUS can be used without the presence of the radiologist on-site while allowing remote evaluation of the images and archiving of the volume data. However, further studies are needed to show its efficacy.